
Do not use the prefix mono- if there is only one atom of the first element. If there is more than one atom of this element in the molecular formula, use a numerical prefix to indicate the number of atoms, as listed in Table 3.6 “Numerical Prefixes Used in Naming Molecular Compounds”. Begin the name with the element name of the first element.This is why you need to know the names and symbols of the elements in Table 3.8 “Names and Symbols of Common Elements”. Identify the elements in the molecule from its formula.

Here, we will start with relatively simple molecules that have only two elements in them, the so-called binary compounds: By following the rules of nomenclature, each and every compound has its own unique name, and each name refers to one and only one compound. The answer is a very specific system of naming compounds, called chemical nomenclature. (Compounds between a metal and a nonmetal are different and are considered in “Ions and Ionic Compounds”.) Furthermore, in some cases there are many different kinds of molecules that can be formed between any given elements, with all the different molecules having different chemical and physical properties. In particular, when nonmetals connect with other nonmetals, the compound typically exists as molecules. You will see other examples of this “ball and cylinder” representation of molecules throughout this book. In Chapter 9 “Chemical Bonds”, we will explore the origin of chemical bonds. This connection is called a chemical bond.
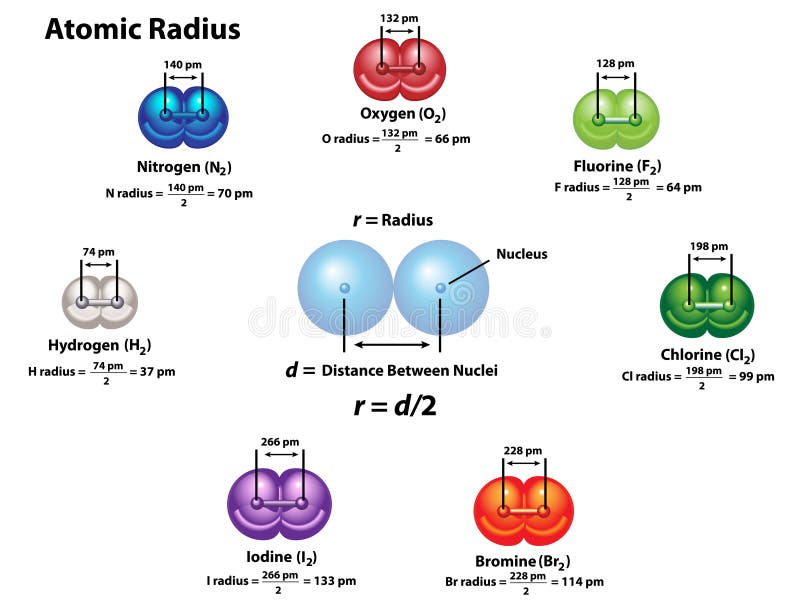
A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a molecule. An atom is represented by a small ball or sphere, which generally indicates where the nucleus is in the molecule. The molecule on the right shows that one form of elemental phosphorus exists, as a four-atom molecule.įigure 3.3 “Molecular Art of S” shows two examples of how we will be representing molecules in this text. It is assumed that there is only one atom in a formula if there is no numerical subscript on the right side of an element’s symbol.įigure 3.3 “Molecular Art of S 8 and P 4 Molecules.” If each green ball represents a sulfur atom, then the diagram on the left represents an S 8 molecule. Otherwise, we will assume that elements exist as individual atoms, rather than molecules. Other elements exist as molecules-for example, sulfur normally exists as an eight-atom molecule, S 8, while phosphorus exists as a four-atom molecule, P 4 (see Figure 3.3 “Molecular Art of S”). Other diatomic elements have similar formulas: O 2, N 2, and so forth. (Sometimes only the word formula is used, and its meaning is inferred from the context.) For example, the molecular formula for elemental hydrogen is H 2, with H being the symbol for hydrogen and the subscript 2 implying that there are two atoms of this element in the molecule. As with any molecule, these elements are labelled with a molecular formula, a formal listing of what and how many atoms are in a molecule. Other elements also exist naturally as diatomic molecules (see the list “Elements That Exist as Diatomic Molecules”).
For example, hydrogen and oxygen exist as two-atom molecules. Some elements exist naturally as molecules. A molecule, however, is composed of more than one atom. In some respects, a molecule is similar to an atom. A molecule is the smallest part of a substance that has the physical and chemical properties of that substance. These multiatom combinations are called molecules. There are many substances that exist as two or more atoms connected together so strongly that they behave as a single particle.



 0 kommentar(er)
0 kommentar(er)
